Latent Heat
Latent Heat: Overview
This topic covers concepts such as melting point of ice, temperature versus heat graph for heating of ice, latent heat of fusion, latent heat of vaporisation, and specific latent heat of fusion.
Important Questions on Latent Heat
Draw the temperature versus heat graph for heating of ice. Describe the phase change of the water on the basis of the graph. Explain the terms specific latent heat of fusion and specific latent heat of vaporization.
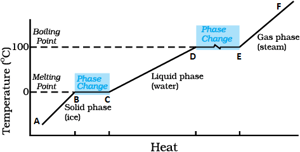
Identify the part representing latent heat of vaporization in the temperature vs heat graph for melting of ice.
The amount of heat energy absorbed at constant temperature by unit mass of a liquid to convert into gaseous phase is called the specific latent heat of _____.
The amount of heat energy absorbed at constant temperature by unit mass of a solid to convert into liquid phase is called the
Observe the following temperature Vs. time graph and fill in the blank:
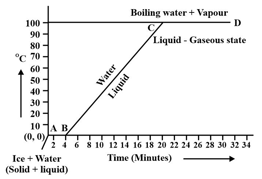
During transition of solid phase to liquid, the object absorbs _____ (electric \chemical \heat) energy, but its temperature does not increase.
Observe the following temperature Versus time graph and fill in the blank.
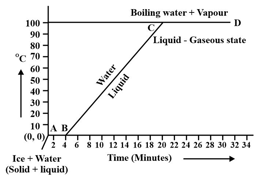
The constant temperature, at which the ice converts into water is called the _____ point of ice.
(Choose from: boiling/melting)
Observe the following temperature Versus time graph and fill in the blank.
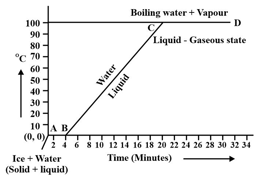
When ice is heated it melts at _____ and converts into water at this constant temperature.
(Choose from: /)
At what temperature solid ice and liquid water coexist together? Write the numerical value in celsius scale.
